The Reaction of 1 Mole of Sulfur Dioxide With Oxygen
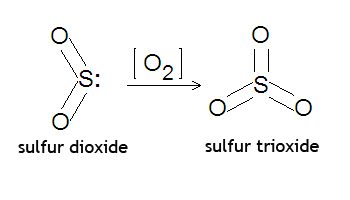
Moles 672 L. Sulfur dioxide reacts with oxygen to form sulfur trioxide according to the equation 2SO2 g O2 g 2SO3 g Samples of sulfur dioxide oxygen and sulfur trioxide were added to a flask of volume 140 dm3 and allowed to reach.

In The Womb National Geographic Worksheet Answer Key Prior To Preaching About In The Womb National Geographic Worksheet Answer Key Remember To Be Aware That
Use the mole ratio to determine the moles of H₂SO₄.

. This is the best answer based on feedback and ratings. 2SO2g O2g 2SO3g Two moles of sulfur will react with 1 mole of oxygen gas to produce two moles of sulfur trioxide. Molar mass of SO2 640638 gmol.
Two important things to notice here. The molecular formula of sulphur dioxide is S O 2. Sulfur oxygen sulfur dioxide.
Apr 19 2015. Sulfur dioxide and oxygen react to form sulfur trioxide as follows 2SO_2g O_2g 2SO_3g 1 mole of sulfur dioxide is mixed with 05 moles of oxygen gas in a 1 L container. Based on the reaction as written water vapor is produced.
Sulfur Dioxide molecular weight. There are 12 x 1024 hydrogen atoms in 1 mole of methane molecules. The reaction kinetics of SO 2 oxidation by oxygen catalyzed by mixtures of MnII and FeIII in aqueous solutions over a wide range of pH from 26 to 65 have been studied at low concentrations of SIV and the metal ions.
Equilibria SCT Page 1 of 16 Q1. See the answer. Calculate the concentration equilibrium.
The reaction of 1 mol of sulfur dioxide with oxygenwhat is the chemical reaction. 100 1 rating 2so. Molar mass of S O 2 3 2.
Sulfur dioxide or SO2 will react with oxygen gas or O2 to form sulfur trioxide or SO3 according to the balanced chemical equation. See the answer See the answer done loading. Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis.
The following rate expression is obtained. That is at STP 1 moles 224 L. The balanced chemical equation stipulates the formation of two moles of sulfur trioxide when two moles of sulfur dioxide and one mole of oxygen react.
From equation above the mole ratio of SO₂H₂SO₄ is 22 11. Sulfur dioxide reacts with oxygen to form sulfur trioxide according to the equation 2SO 2 g O 2 g 2SO 3 g a Write an expression for the equilibrium constant K c for this reaction and deduce its units. By cross multiplication 672 L x 1 moles 224 L 3 moles.
The standard enthalpy change of reaction carries a negative sign. 32065 1599942 Percent composition by element. 0 6 g So mass of 1 m o l e of S O 2 6 4.
The equation should read. Your teacher will not demonstrate this reaction because the sulfur dioxide that forms is a poisonous gas that you and your classmates should not be exposed to. Previous question Next question.
Convert grams Sulfur Dioxide to moles or moles Sulfur Dioxide to grams. Sulfur dioxide and molecular oxygen react to form sulfur trioxide. 0 6 2 1 6 6 4.
The fact that the standard enthalpy change of. Mass of one mole of FeSO 4 152 g number of moles of FeSO 4 used g g number of moles of Fe 2O 3. Sulfur dioxide gas SO2 reacts with excess oxygen gas O2 and excess liquid water H2O to form liquid sulfuric acid H2SO4.
The catalytic synergism between the two catalysts as a function of MnII. In the laboratory a chemist carries out this reaction with 672 L of sulfur dioxide and gets 250 g of sulfuric acid. If 674 x 10 22 molecules of oxygen are consumed in the reaction how many moles of sulfur trioxide were produced.
Sulfur burns in oxygen to form sulfur dioxide. In 1802 grams of H2O there is one mole of oxygen atoms. 2SO2 g O2 g 2SO3 g ΔH rxn 198 kJmol.
View the full answer. The reaction is usually carried out at a temperature between. In the reaction 2SO2 O2 2SO3 025 mole of sulfur dioxide is mixed with 025 mole of oxygen and allowed to react.
A video on sulfur burning in oxygen. The composition of the reaction mixture is 1 volume of sulfur dioxide to 1 volume of oxygen. An industrial chemist studying this reaction fills a 750 L tank with 43 mol of sulfur dioxide gas and 72 mol of oxygen gas and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 30 mol.
There is 09 mol of SO_3 at equilibrium. 2 moles of methane will produce 4 moles of CO2 molecules. FeIII has been confirmed.

Two Chemical Reactions Are Given I Sulphur Dioxide Combines With Oxygen To Form Sulphur Trioxide Ii Sodium Reacts With Water A Write The Balanced Chemical Equation For Any One Of The Above Reactions B Which

No comments for "The Reaction of 1 Mole of Sulfur Dioxide With Oxygen"
Post a Comment